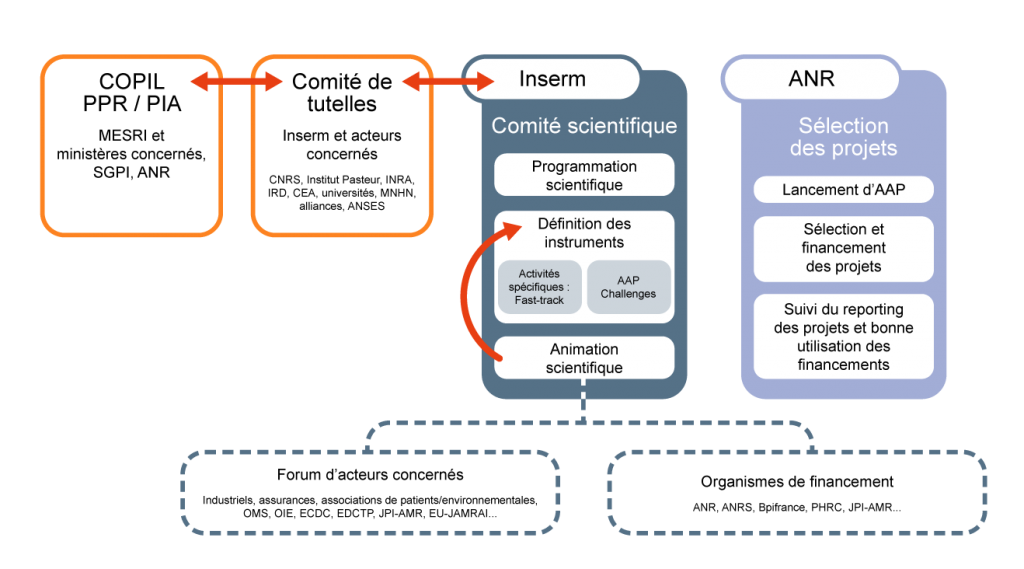
The governance of the Antibioresistance RPP will be organized around three main bodies, whose roles and missions are defined below:
- The Comité de tutelles, chaired by the Chairman and CEO of Inserm, will bring together the major players and institutional partners involved in the field, including CNRS, Institut Pasteur, IRD, Inra, CEA, Anses, Université de Paris, Université Lyon 1, MNHN and the alliances, and will meet at least once a year, and more frequently during the set-up phase. This committee will report on the progress of the PPR to the PPR/PIA Steering Committee (COPIL), made up of representatives from the Ministries of Higher Education, Research and Innovation (MESRI), Solidarity and Health, Agriculture and Food, Ecological Transition and Solidarity, Europe and Foreign Affairs and other relevant ministries, the General Secretariat for Investment (SGPI), and the National Research Agency (ANR). This committee will also liaise with the permanent interministerial committee on antibiotic resistance, coordinated by the Ministry of Solidarity and Health. As with other RPPs, the role of this COPIL is to outline strategies and decide on the allocation of resources based on proposals put forward by the Scientific Committee and validated by the Trustees Committee. The composition of the Supervisory Committee may change according to the needs of the program.
- The Scientific Committee (SC) will be set up by Inserm as soon as the operational phase of the PPR begins. It will be chaired by a recognized scientist in the field, and will include, among others, academic scientific experts. This committee will ensure effective coordination with the relevant ministries, French institutes and universities, and international initiatives dedicated to anti-biotic resistance research. At least one third of the SC members will be experts working in other countries. As the guarantor of the RPP’s ambition and leverage effect, the SC’s main missions will be to:
- identify and define scientific research priorities within a “One Health” approach, and draw up the scientific program;propose funding instruments (calls for projects – AAP, calls for expressions of interest – AMI, “emergency” equipment and funding, etc.);organize scientific leadership, in collaboration with the French National Research Agency (ANR), including forward-looking thinking and communication;provide scientific and forward-looking support for consortiums.
- a forum of stakeholders including industrialists, insurance companies, patient and environmental associations, WHO, OIE, ECDC, EU-JAMRAI, etc. Discussions will be initiated with the players in this forum to create a forum of co-funders, so as to seize: 1) opportunities for collaboration between researchers/players in the private and academic sectors, 2) investments from the private sector to encourage support for the development and marketing of innovative products developed by the PPR, and 3) to strengthen the presence of French academic teams in European IMI program calls for projects;
- bodies offering funded programs on antibiotic resistance (ANR, ANRS, PHRC, PREPS, Bpifrance, ARIIS, JPI-AMR, etc.).
- The ANR will implement the research programs identified by the Board of Directors and validated by the COPIL. For this function, the agency is responsible either for setting up the financing of actions that will not be the subject of a call for projects, or for setting up calls for projects for actions that require it: 1) launch of the AMI(s) 2) selection of projects, 3) contractualization and financing of projects, reporting on projects and the proper use of financing.