Antimicrobial resistance (AMR), a ‘silent’ or ‘overlooked’ pandemic, 1,2 is classed as a threat to global health and development by the World Health Organization (WHO). If left unchecked, it could cause 10 million deaths per year by 2050,3 cost the global economy US$100tn,1 and drive 28 million people into extreme poverty.4 This cannot be overlooked, and we cannot be silent — the development of novel antimicrobials must be a public health priority.
Decisive political leadership, guided by expert opinion, is vital to navigating public health crises. The COVID-19 pandemic has shown that coordinated action is possible on national and international scales. However, the focus on SARS-CoV-2 has led to a substantial decline in public awareness of AMR and its hazards.
AMR disproportionately affects those in low- and middle-income countries (LMIC),3,5 where the lack of means for effective prevention, diagnostics and treatment of infectious diseases is combined with over-the-counter access to antibiotics. In South Asia and Latin America where access to second-line antibiotics is good, the AMR burden is due to high levels of resistance.6 Global collaborations such as the Quadripartite collaboration7 and the Global Leaders Group8 are promoting a One Health approach to increase global awareness and surveillance of AMR a on an international scale.
One Health – the problem and the cure
The One Health concept aims to sustainably balance and optimize the health of people, animals and ecosystems. In the context of AMR, it is a multidisciplinary and multi-sectorial approach to preventing emergent and resurgent infectious diseases.
Potential routes to AMR development are complex and linked to all OneHealth sectors (Figure 1 & Publications Office (europa.eu)), for example:
- La surutilisation et le mauvais usage des antimicrobiens dans les milieux médicaux et vétérinaires,9
- L’utilisation non réglementée et non contrôlée d’antimicrobiens en agriculture,10 entraînant en particulier une résistance aux antifongiques,11
- Les réservoirs environnementaux d’antibiorésistance (par exemple, les eaux usées et les déchets non traités qui atteignent les réservoirs d’eau).5
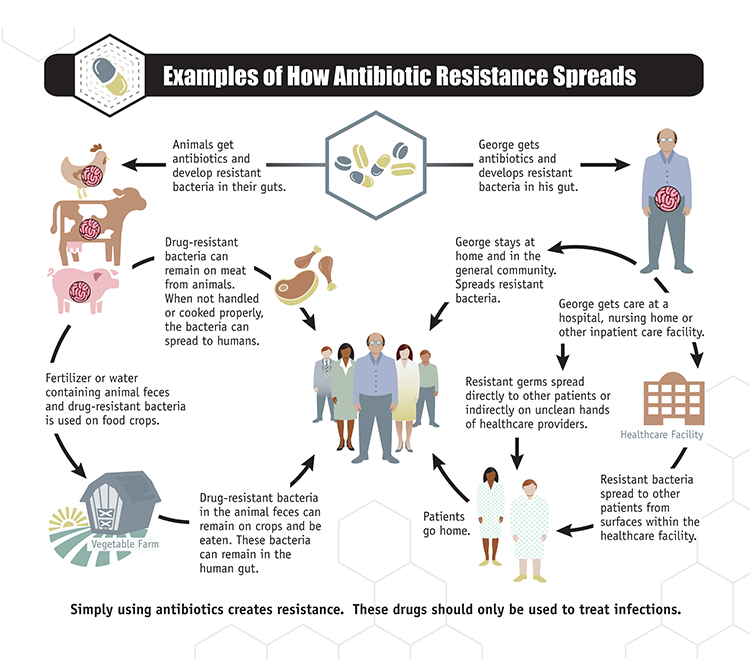
The One Health approach embraces a variety of AMR-countering strategies both scientific and logistical:
- Le développement de nouveaux antimicrobiens, d’outils de diagnostic précis et rapides, de vaccins et d’immunothérapies efficaces,
- Une compréhension approfondie de l’antibiorésistance au niveau moléculaire, des interactions cible-médicament, de l’immunologie des maladies infectieuses et de la dysbiose suite à un traitement antibiotique,
- L’amélioration des programmes de gestion des antimicrobiens et de prévention des infections, l’efficacité des programmes actuels étant insuffisante,12
- Amélioration de l’accès aux ressources existantes en soins de santé, une meilleure gestion de la chaîne d’approvisionnement d’antimicrobiens et une réduction de l’exposition environnementale aux résidus antibiotiques,
- Amélioration de la surveillance en santé publique et de la formation du personnel de santé au niveau mondial.
The essential component: Funding
Successful innovation relies on resource mobilization, but at present, antimicrobial R & D is fragmented, with a lack of collaboration between academia, industry and clinicians. CARB-X (https://carb-x.org) and the AMR Action Fund (https://www.amractionfund.com) are good examples of essential funding sources for novel antimicrobial development. Further funding by both public and private investors needs to be highly visible to prove that antimicrobial R & D is a global priority.
Interruptions to R & D funding can lead to abandonment of projects, new firms failing to grow into mature businesses, and researchers in both academia and industry moving on to more financially rewarding areas of research. The collapse of companies that successfully brought new antibiotics to market, yet still suffered bankruptcy (e.g., Melinta Therapeutics and Achaogen), contribute to the lack of confidence for investment in antimicrobial R & D.
In response, funding schemes have been launched in the UK13 and the USA14 to reward firms that launch new antimicrobials through an annual fixed fee, delinking financial reward from the volume of prescriptions. To encourage international pharmaceutical companies to re-invest in antimicrobial R&D, similar schemes need to be offered globally.
In addition to R&D funding, investment in the AMR workforce is necessary. Central governments can establish early career fellowships in AMR, provide training in high risk antimicrobial therapeutic and prophylactic innovation, and establish centres for AMR research (national/international) that would act as hubs for R&D.
Conclusion
To tackle AMR globally will require an integrated, collaborative and multi-sector response to AMR, substantial and sustained funding along with decisive leadership and resolute support from governments, global leaders, academia, industry and the not-for-profit sector. Political initiatives on AMR need to be bold, innovative, coordinated and sustainable.
References
1. Mendelson M, Sharland M, Mpundu M. Antibiotic resistance: Calling time on the ‘silent pandemic’. JAC Antimicrob Resist 2022; 4:dlac016
2. Laxminarayan R. The overlooked pandemic of antimicrobial resistance. Lancet 2022;399:606–7.
3. O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance 2016.
4. World Bank Group. Drug-resistant infections: a threat to our economic future 2017.
5. World Health Organization. Technical brief on water, sanitation, hygiene and wastewater management to prevent infections and reduce the spread of antimicrobial resistance 2020.
6. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022;399:629–55.
7. Quadrapartite Collaboration. One health joint plan of action working together for the health of humans, animals, plants and the environment 2022.
8. Global Leaders Group on AMR. Priorities of the global leaders group on AMR for 2021-2022 2021.
9. McEwen SA, Collignon PJ. Antimicrobial resistance: a one health perspective. Microbiol Spectr 2018;6.
10. Taylor P, Reeder R. Antibiotic use on crops in low and middle-income countries based on recommendations made by agricultural advisors. CABI Agric Biosci 2020;11:1–14.
11. Miller SA, Ferreira JP, LeJeune JT. Antimicrobial use and resistance in plant agriculture: A one health perspective. Agriculture, 12(2):289.
12. Poluektova O, Robertson DA, Rafferty A, Cunney R, Lunn PD. A scoping review and behavioural analysis of factors underlying overuse of antimicrobials. JAC Antimicrob Resist 2023; 4:dlad043,
13. Mahase E. UK launches subscription style model for antibiotics to encourage new development. BMJ 2020;369:m2468.
14. Press release: Bennet, Young, Doyle, Ferguson Introduce PASTEUR Act to Fight Antimicrobial Resistance 2021.
Auteurs

Derry Mercer
Responsable du Programme Antimicrobiens/Antimicrobial Programme Head

Cyril Guyard
Directeur Scientifique, Direction Scientifique et technologique/Chief Scientific Officer
Article edited by Loane Serrano

BIOASTER, le premier Institut d’Innovation Technologique en Microbiologie. Favoriser, accélérer et apporter une nouvelle valeur ajoutée aux projets collaboratifs, avec des partenaires publics et privés, pour mieux répondre aux enjeux de santé globale.